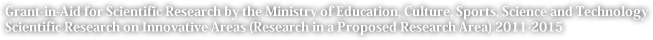Home > Researcher Columns > The Day Oxygen Appeared on Earth
The Day Oxygen Appeared on Earth
“Like the air” is a common expression. It describes something which is important and essential, but which we pay no attention to in our everyday lives. According to internet dictionaries, it has many negative meanings describing “something with no sense of presence, and which produces no difference by its presence or absence.” However recently it has adopted some positive nuances, in the sense of describing “something necessary for life.” In the case of the line, “You are like the air to me,” a person who does not correctly understand which meaning “the air” implies is certainly heading for trouble.
To be sure, air is an essential element which we require to continue living. The air is a gas that composes the lowest layer of the Earth’s atmosphere, and is chemically composed primarily of nitrogen and oxygen. We breathe using the oxygen in the air to gain the energy needed for biological activities, and in this sense the air that we require is specifically oxygen. However would you be surprised to learn that this “like the air” oxygen became a primary component of the atmosphere only relatively recently on the 4.6 billion year timescale of the Earth’s history?
The oxygen in the atmosphere is produced by oxygen-producing photosynthetic organisms (oxygenic phototrophs) such as cyanobacteria and phytoplankton. Therefore naturally the Earth had no oxygen before this kind of life emerged. Estimates based on a variety of geological evidence place the appearance of oxygen in Earth’s atmosphere at 2.0 – 2.3 billion years ago (Figure 1). This means that there was almost no oxygen in the atmosphere for the first half of Earth’s history. The concentration of atmospheric oxygen which rose 2.0 – 2.3 billion years ago first stabilized at approximately 1/100 of the current level. Then 500 - 600 million years ago, a second jump occurred and oxygen became a primary component of the atmosphere at its current level of 20%. If all of Earth’s history is considered to be a single year, with the Earth born on January 1 and the present time being December 31, then this second jump occurred in late November.
So why did the concentration of oxygen in the atmosphere increase so dramatically at specific times? In simple terms, one might expect it to be because the phototrophs which produce oxygen appeared 2.0 – 2.3 billion years ago. However it is not that simple. When we look at the geological record, it appears that primitive oxygenic phototrophs such as cyanobacteria appeared a minimum of 2.7 billion years ago. So if oxygen-producing creatures already existed, why did oxygen not build up in the atmosphere before 2.3 billion years ago? And why did the oxygen concentration rise suddenly 2.3 billion and 6 billion years ago?
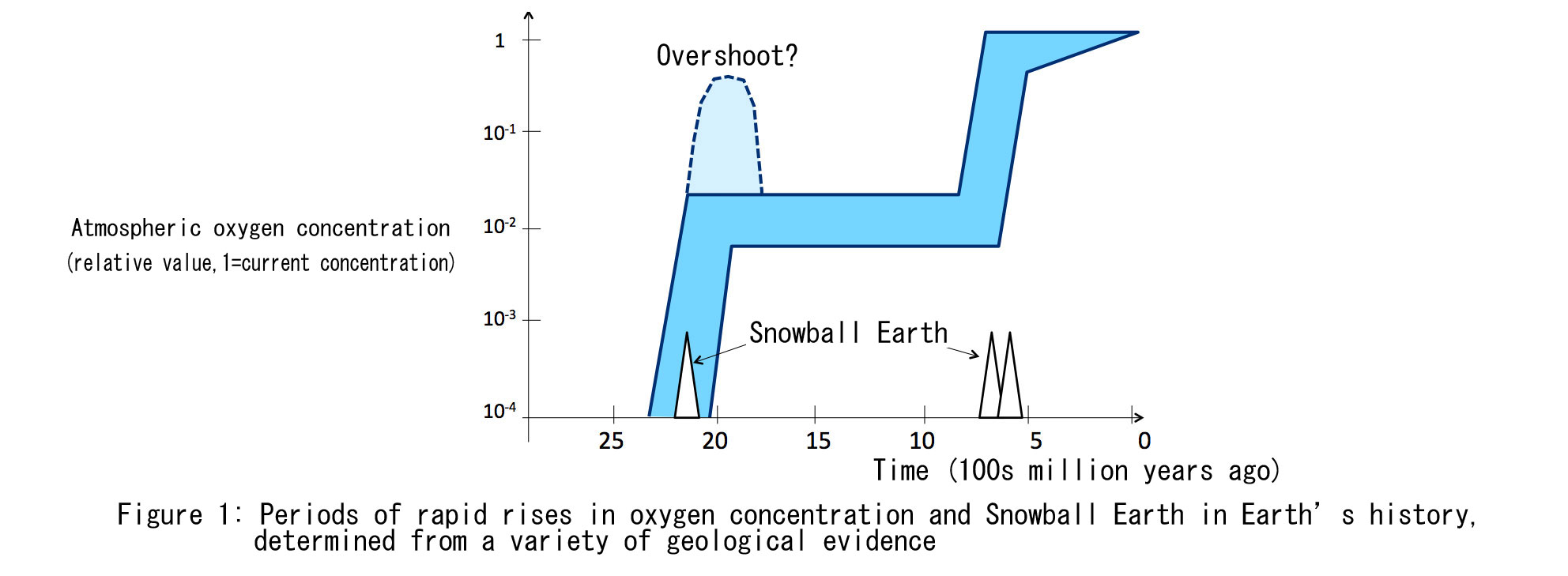
In fact, the concentration of oxygen in the atmosphere is determined by more than just the biological activities that produce it. The key is the balance with the supply of reducing substances (such as bivalent iron and methane) that consume the oxygen produced in the atmosphere and oceans. It is the same principle which explains why the amount of money one saves each month varies when the income remains the same and the expenditures vary. Many researchers also think that different atmospheric oxygen concentrations are possible when the amount of oxygen produced and the amount of oxygen consumed are in balance. This can be compared to two persons who have the same income; one lives in a large but energy-efficient home, while the other lives in a small but energy-inefficient home. Both end up with the same level of expenditures. Even when the income and expenditures of both persons are exactly the same, the apparent conditions are different.
This kind of state is called a “multistable state,” and it is believed that such states exist not only in the Earth, but also in mineral crystals, chemical chain reactions, ecosystems, neural networks in the brain, and other complex systems. In the case of the Earth, from a long-term perspective, the Earth receives a constant amount of energy from the sun, and maintains a stable balance between oxygen generation and consumption on the surface. However the stable state that we currently see is only one of many stable states, and when a large external disturbance occurs, this state can change from one stable state to another stable state (Figure 2). This means that the stable periods and sudden rises in the oxygen concentration over Earth’s history can be understood as multistable states and the transitions between them.
So in the case of oxygen in the Earth’s atmosphere, what is the cause of this kind of transition between stable states? A clear answer to this question has not yet been found, however our research group believes that “Snowball Earth may be the cause. Snowball Earth refers to a geological event thought to have occurred 2.2 – 2.3 billion years ago and again 600 – 700 million years ago, in which the entire surface of the Earth was literally frozen. In order to escape from the frozen conditions, it was necessary that a large amount of carbon dioxide accumulate in the atmosphere. As a result, the Earth immediately after the freeze was for a time an extremely warm place. Under this kind of super greenhouse condition, cyanobacteria became extremely active, producing a large amount of oxygen. This kind of extreme climate change and the resulting production of oxygen may have caused a transition between multistable states. To continue the metaphor, this is like the person from before who lived in the small, energy-inefficient house winning the lottery and moving into a large, energy-efficient house. Of course, the boundary conditions of income and expenditures do not change.
In this case, a climate change such as snowball Earth which appears to have wiped out nearly all life may in fact have played an important role in forming the oxygen-rich atmosphere which is essential to the explosion of diverse life that later occurred. The current 20% oxygen concentration is stable, however there is the possibility that it may transition to a different stable state in the future. One way of finding answers to these questions may be observation of terrestrial planets outside the solar system. If we find a second and a third Earth beyond the solar system and understand the diversity and universality of the atmospheric oxygen concentrations, we may have more appreciation for our “like the air” oxygen atmosphere.
Yasuhito Sekine (University of Tokyo)